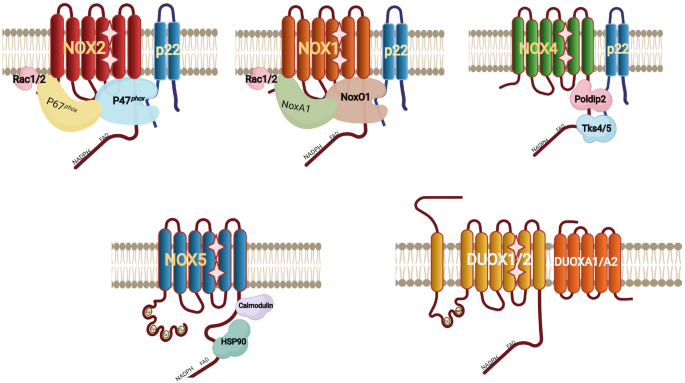
Direct Synthesis of α-Iodoenones by IPy2BF4-Promoted Rearrangement of Propargylic Esters | The Journal of Organic Chemistry

Reductive Cyclization of o-Nitroarylated-α,β-unsaturated Aldehydes and Ketones with TiCl3/HCl or Fe/HCl Leading to 1,2,3,9-Tetrahydro-4H-carbazol-4-ones and Related Heterocycles | The Journal of Organic Chemistry

A Palladium-Catalyzed Ullmann Cross-Coupling/Reductive Cyclization Route to the Carbazole Natural Products 3-Methyl-9H-carbazole, Glycoborine, Glycozoline, Clauszoline K, Mukonine, and Karapinchamine A | The Journal of Organic Chemistry

Conversion of the Enzymatically Derived (1S,2S)-3-Bromocyclohexa-3,5-diene-1,2-diol into Enantiomerically Pure Compounds Embodying the Pentacyclic Framework of Vindoline | The Journal of Organic Chemistry

Reductive Cyclization of o-Nitroarylated-α,β-unsaturated Aldehydes and Ketones with TiCl3/HCl or Fe/HCl Leading to 1,2,3,9-Tetrahydro-4H-carbazol-4-ones and Related Heterocycles | The Journal of Organic Chemistry

Conversion of the Enzymatically Derived (1S,2S)-3-Bromocyclohexa-3,5-diene-1,2-diol into Enantiomerically Pure Compounds Embodying the Pentacyclic Framework of Vindoline | The Journal of Organic Chemistry

Conversion of the Enzymatically Derived (1S,2S)-3-Bromocyclohexa-3,5-diene-1,2-diol into Enantiomerically Pure Compounds Embodying the Pentacyclic Framework of Vindoline | The Journal of Organic Chemistry
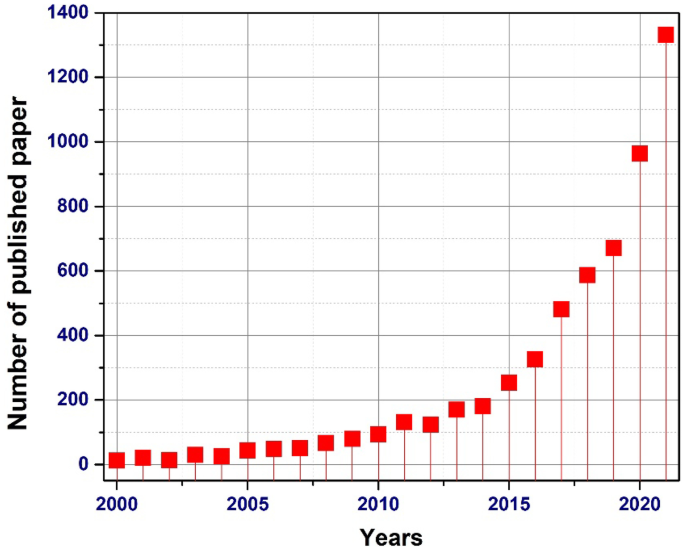
Recent trends of silicon elastomer-based nanocomposites and their sensing applications | SpringerLink

Conversion of the Enzymatically Derived (1S,2S)-3-Bromocyclohexa-3,5-diene-1,2-diol into Enantiomerically Pure Compounds Embodying the Pentacyclic Framework of Vindoline | The Journal of Organic Chemistry

A Palladium-Catalyzed Ullmann Cross-Coupling/Reductive Cyclization Route to the Carbazole Natural Products 3-Methyl-9H-carbazole, Glycoborine, Glycozoline, Clauszoline K, Mukonine, and Karapinchamine A | The Journal of Organic Chemistry